
Ultraviolet (UV) light is one form of electromagnetic energy produced naturally by the sun. UV is a spectrum of light just below the visible light and it is split into four distinct spectral areas – Vacuum UV (100 to 200 nm), UVC (200 to 280 nm), UVB (280 to 315 nm) and UVA (315 to 400 nm).
The entire UV spectrum can kill or inactivate many microorganism species, preventing them from replicating. UVC energy centred around 254 nanometres provides the most germicidal effect. Hence UVC is also known as Ultraviolet Germicidal Irradiation (UVGI).
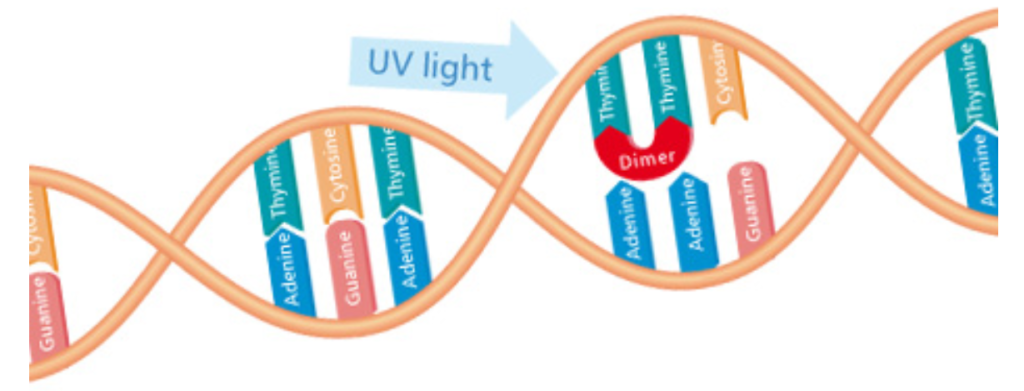
Dimerization or Altering of the Molecular Bonds of a Bacteria’s DNA by UVGI.
Picture Source: Steril Innovations
UGVI exposure inactivates microbial organisms such as bacteria and viruses by altering the structure and the molecular bonds of their DNA (Deoxyribonucleic acid). DNA is a “blue print” these organisms use to develop, function and reproduce. By destroying the organism’s ability to reproduce, it becomes harmless since it cannot colonize. After UVGI exposure, the organism dies off leaving no offspring, and the population of the microorganism diminishes rapidly.
Ultraviolet germicidal lamps provide a much more powerful and concentrated effect of ultraviolet energy than can be found naturally. Such lamps sanitize air that is passed directly in their path to destroy pathogens that come in contact with the UV rays. Musty, mouldy odours can be eradicated, along with airborne disease threats, including emerging diseases like SARS virus, species-jumping diseases like avian flu and multi-drug resistant bacterial strains (e.g. MRSA) commonly found in hospitals.
How UV Lamps Work

Picture Source: Steril-Aire

Picture Source: 2008 ASHARE Handbook
Today UVGI is artificially made using specialized lamps producing UVGI at 254 nm.
UVC tubes are filled with argon and mercury gas with electrodes on either end. A high-voltage electric current runs through the gas between the electrodes. When one of the electrons from that current strikes a mercury molecule, part of its energy is absorbed, exciting the mercury. The mercury then shoots out the energy as a photon of ultraviolet light. Although UVGI is invisible to the human eye, small amounts of energy released at visible wavelengths produce the blue glow commonly associated with UVC lamps.