About Photocatalysis
What is a Photocatalyst?
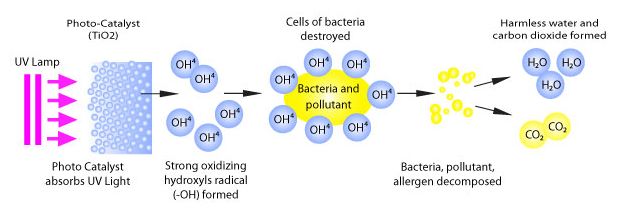
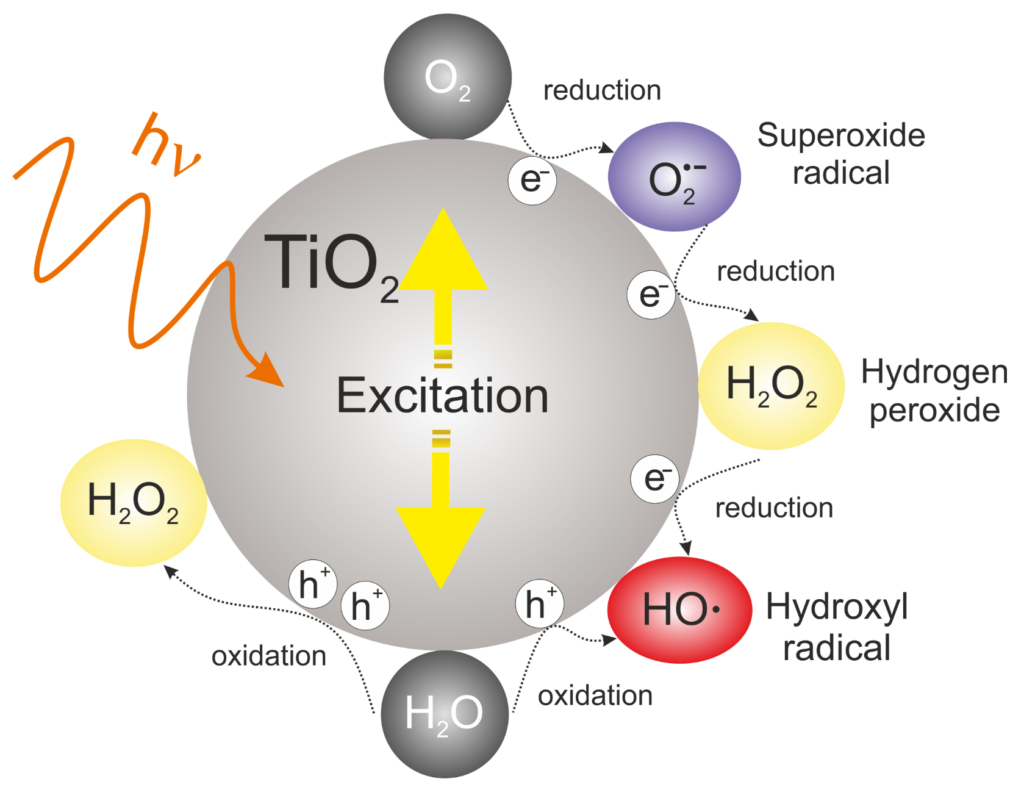
A photocatalyst is a substance that uses light energy to facilitate a chemical reaction. Titanium dioxide (TiO2) is a photocatalyst. When exposed to enough ultraviolet (UV) light, titanium dioxide can induce the transformation of harmful substances and toxic compounds into benign constituents such carbon dioxide (CO2) and water (H2O).