About Fuel Cells
What is a Fuel Cell?
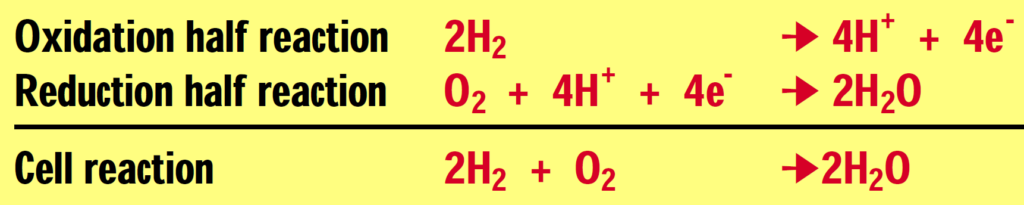
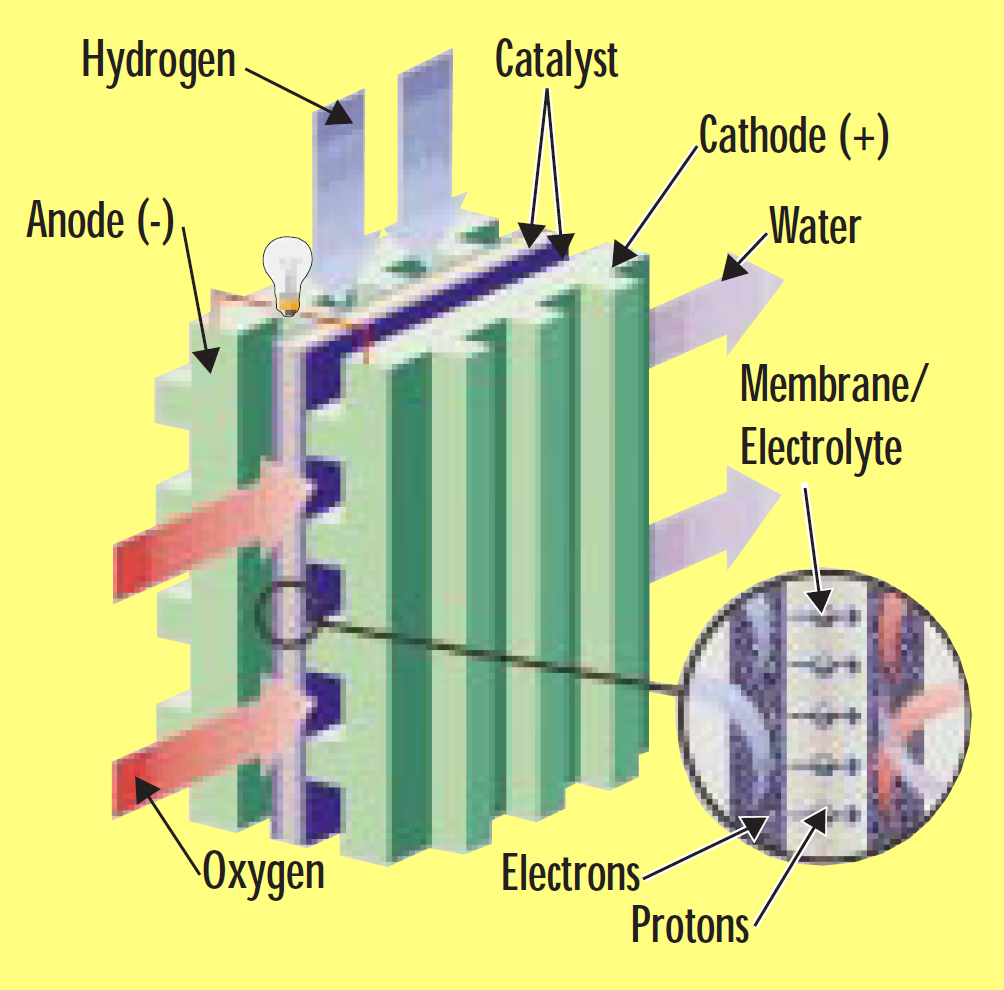
A fuel cell is an electrochemical device that combines hydrogen and oxygen to produce electricity, with water and heat as the by-products. A fuel cell is similar in structure to a battery but it does not run down, nor does it require recharging — as long as hydrogen is supplied, it will continue to operate. The conversion of the fuel (hydrogen) to energy takes place without combustion; therefore the process is highly efficient, clean and quiet.